What’s Arexvy? After decades, US authorizes first RSV vaccine 2023
The US has cleared the first vaccine for people 60 and older to protect against respiratory syncytial virus (RSV).
The Food and Drug Administration (FDA) gave Arexvy permission to be used in the country on Wednesday.
It is said that the vaccine will protect older people from the lung virus.
Now, the Centers for Disease Control and Prevention (CDC) must decide if every senior really needs RSV protection or only those who are thought to be at high risk for RSV.
The Advisory Committee on Immunization Practices (ACIP) will decide in June 2023 how the vaccine should be used in the United States.
RSV is a “highly contagious” virus that causes illnesses in the lungs and airways.
It’s very common, and it’s easy to get when you cough or sneeze. The NHS says that by the time a child is two years old, almost all of them have had it.
RSV can cause a cough or cold in older children and adults, but it can cause bronchiolitis in young children. This is a common but sometimes dangerous chest illness.

The CDC said that most people who get RSV have a mild sickness that lasts between one and two weeks.
Some people, on the other hand, may have a more serious RSV illness.
About Arexvy, what you need to know
Arexvy was cleared by the FDA to protect people over 60 from RSV-related diseases of the lower respiratory system.
After its third study, the pharmaceutical company GSK said, “The vaccine showed statistically significant and clinically meaningful overall efficacy against RSV-LRTD in adults age 60 and older.”
The company also said that the vaccine was “well tolerated in general and had an acceptable safety profile.”
After looking at how safe and effective the vaccine was, the FDA said, “The safety and effectiveness of Arexvy are based on the FDA’s analysis of data from an ongoing, randomised, placebo-controlled clinical study being done in the U.S. and internationally on people 60 years and older.”
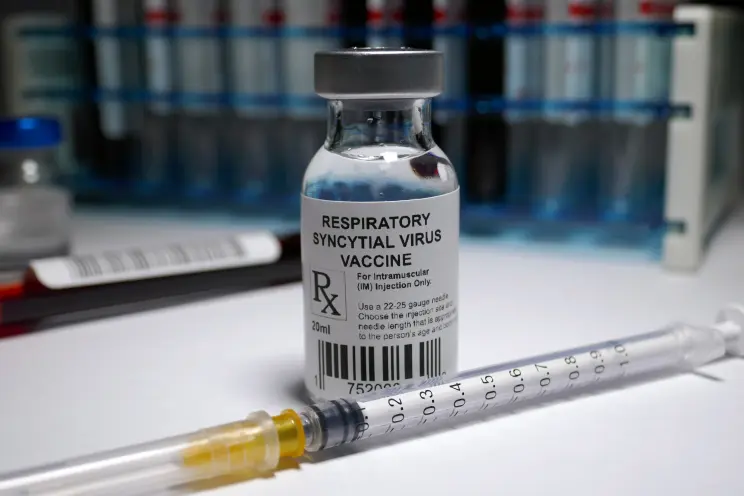
GSK’s senior vice president and global head of vaccines research and development, Dr. Phil Dormitzer, said that the company is already making doses of the vaccine.
How come it has taken so long to make a vaccine?
In the 1960s, there was a big loss when an experimental shot made illnesses in children worse.
Scientists have now found a better way to make these vaccines, however.
GSK’s new vaccine for older people helps the immune system learn to recognize a protein on the surface of RSV. It also has an ingredient that makes the immune system’s response stronger.
Clinical studies
In a clinical study of Arexvy, the safety and usefulness of a single amount given to people 60 and older will be looked at.
Participants will stay in the study for three RSV seasons, or three years, to find out how long the vaccine works and how safe and successful it is to get it more than once.
The FDA has now looked at the results from the first RSV season of the study for a single dose of Arexvy.
About 12,500 people got the vaccine, and the same number got a fake medicine (called a “placebo”).
People who got the vaccine had an 82% lower chance of getting RSV-related lower respiratory tract disease (LRTD) and a 94% lower chance of getting RSV-related LRTD that was serious.
How does it make you feel?
Based on the research, it was found that the vaccine had few side effects.
But people who took Arexvy most often experienced the following side effects:
- Fatigue •
- Muscle pain •
- Headache •
- Joint stiffness •
- Joint paint
The FDA found that people who got the vaccine were more likely to have atrial fibrillation (an uneven heartbeat) than people in the control group.
It also found a possible link between the vaccine and one case of Guillain-Barré syndrome, a rare neurological disease that harms nerve cells and makes muscles weak or paralyzed.
Dr. Dormitzer said it’s hard to know what to think about the Guillain-Barré case, but he added, “At this point, I wouldn’t say it’s a big worry.”
“A big step forward for public health”
Director of the FDA’s Center for Biologics Evaluation and Research Peter Marks said that approving the vaccine is “an important public health achievement to prevent a disease that can be life-threatening.”
He also said, “Older adults, especially those who already have health problems like heart or lung disease or a weak immune system, are at high risk of getting very sick from RSV.”
What are some signs that you might have RSV?
According to the CDC, people usually show signs four to six days after getting sick. Some of these symptoms could be:
- A runny nose
- A loss of appetite
- Coughing
- Sneezing
- Fever
- Wheezing
- Loss of appetite.
On the CDC website, it said that these signs “usually don’t happen all at once, but in stages.”
It also said, “In very young babies, the only symptoms of RSV may be irritability, less activity, and trouble breathing.”
