Study shows gut microorganisms swap antibacterial resistance genes 2023
Antimicrobial resistance occurs when organisms like bacteria become resistant to antimicrobials. UK researchers found the mechanism behind antibiotic resistance and plan to use it to build new drugs.
Antibiotic abuse has led to drug-resistant microorganisms. Antibiotic resistance makes microorganisms propagate more easily.
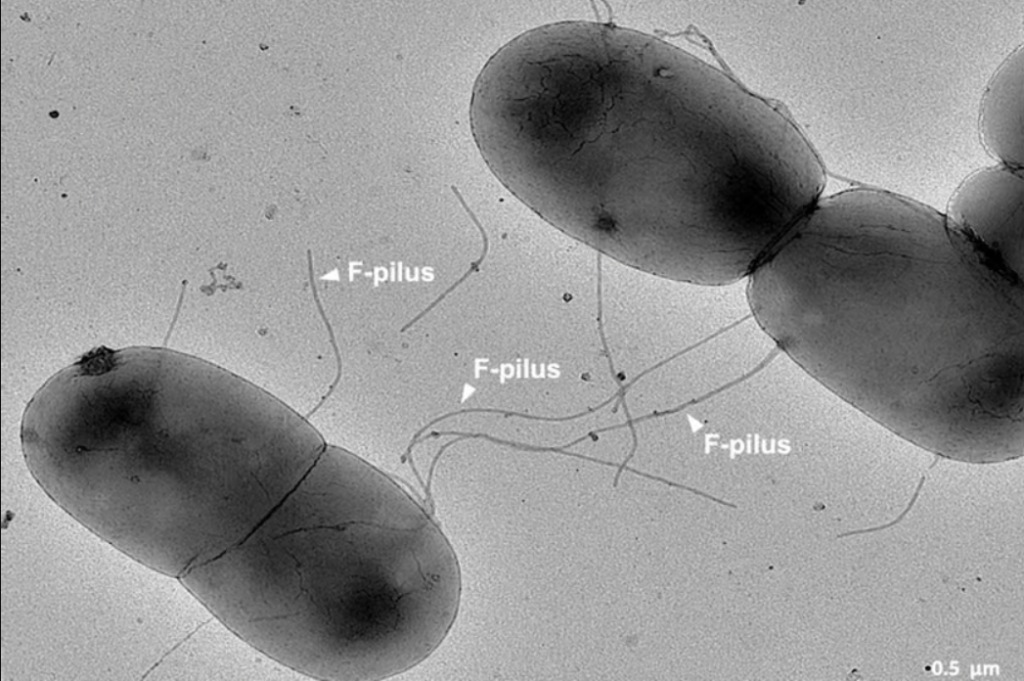
Gut bacteria use F-pili to conjugate and transmit AMR genes. The gut’s acidity, heat, and turbulence were thought to damage F-pili, making gene transfer harder.
The protrusions that bacteria in the gut use to communicate and trade genes for resistance to antibiotics
However, Imperial College London researchers found that F-pili’s ability to transfer AMR genes is unaffected by intestinal circumstances and boosted by their chemical structure.

“The death toll from antimicrobial resistance is expected to match cancer by 2050, meaning we urgently need new strategies to combat this trend,” said research main author Jonasz Patkowski. Bacteria switch genes to propagate resistance, thus knowing this mechanism could lead to new strategies to stop it.
The researchers shook samples of Escherichia coli (E. coli), a common large intestine bacterium, while the F-pili conjugated to test the notion that F-pili are fragile when disturbed. Agitation improved bacterial gene transfer. Importantly, shook bacteria formed a biofilm, a “fence” that protected inside bacteria from antibiotic chemicals.
Researchers employed “molecular tweezers” to pull on the end of the F-pili, which sprung back without breaking. F-pili withstood sodium hydroxide (caustic soda), urea, and 212 °F (100 °C).
F-pili are subunits of interconnected phospholipid molecules, but researchers wanted to better understand their molecular composition. Researchers conducted the pull test with phospholipid-free F-pili. Phospholipid-free F-pili disintegrated easily, showing how crucial these subunits are to structural strength and flexibility.
The work illustrates how F-pili have altered to transfer AMR genes more efficiently and effectively
“Making F-pili is very costly to the bacteria in terms of resources and energy, so it’s no surprise they are worth the effort,” said corresponding author Tiago Costa. “We have shown how F-pili accelerate antibiotic resistance and biofilm formation in turbulent environments, but now we must find ways to combat this very efficient process.”
Now that researchers understand F-pili’s strengths and shortcomings, the structures could be exploited to design new drug delivery systems.
“It’s hard to find a tubular appendage with such strong properties,” Patkowski added. “Bacteria use it to transfer genes, but if we could mimic these properties, we could use similar structures to precisely deliver drugs to the body.”
