Vaccine Research: Global Trends 2023

Vaccine development has dropped from 8-10 years to 260 days in two years. The industry now targets a 60-day turnaround. Post-COVID-19, the sector can swiftly create medicines for infectious diseases, oncology, uncommon disorders, CNS, autoimmune diseases, and more.
Novotech recently convened vaccine industry executives to discuss lessons gained and the future of vaccine development.
The panel:
- Sushant Sahastrabuddhe, Associate Director General, International Vaccine Institute, Korea.
- Clinical Director of Infectious Diseases at Mater Health Services and Associate Professor at the University of Queensland Medical School, Paul Griffin.
- Novotech Senior Consultant Babaji Yadav.
- Novotech Philippines Clinical Services Director Jenny Arellano.
COVID-19 has accelerated global vaccine trials, FDA approval, and advanced manufacturing, according to the panelists. Most crucially, governments and regulatory bodies have permanently accepted many of these innovative approaches, speeding up vaccine development and responding to health issues.
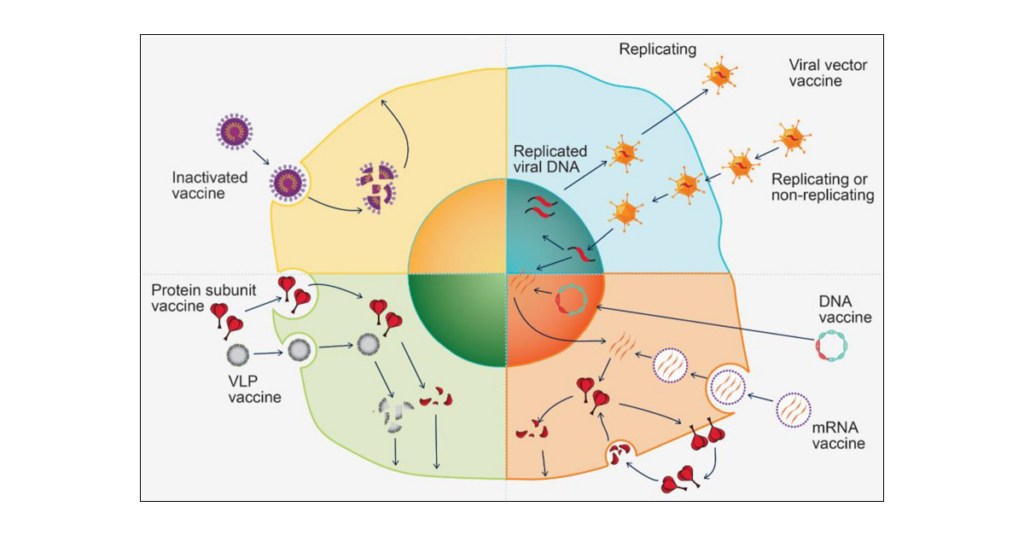
The rapid development of mRNA technology has sped vaccine research, and the plug-and-play approach promises exponential expansion at cheap cost.
Intranasal and patch delivery systems, many of which are self-administered, are already emerging, the panelists said.
Development Partnerships
Jenny Arellano prioritizes vaccine development by carefully selecting partners with a broad footprint across regions and knowledge bandwidth to assist the medication development program locally and globally.
Regulators’ Permanent Changes
Dr. Sushant Sahastrabuddhe said, “One positive point that we have seen during COVID-19 is the way the regulatory agencies showed flexibility in reviewing the protocols, giving clear guidance to the manufacturers, developers, and governments, and having a very clear strategy in terms of how and when to license the products.”
Jenny Arellano said: Asia Pacific regulatory authorities have welcomed quick vaccination assessment processes like the rest of the world. “Regulatory and ethics bodies quickly set up an infrastructure to review clinical trial applications during the pandemic. For COVID-19, regulatory bodies in Asia Pacific have opened the doors for speedy assessment and hired technical experts to assist.
New clinical trial methods—remote monitoring
Dr. Sushant Sahastrabuddhe said “almost 75% of our research was on COVID-19 vaccine” till last year. Vaccines were deprioritized. I think vaccine priorities are back. Diversifying again on global public health diseases.”
She suggested remote monitoring will be used more in vaccine trials.

Some sponsors and CROs like Novotech use this option. During COVID-19, we found we could do more remotely than before.”
Delivery Methods
“I really think intranasal vaccines are going to be an important part of not only what we do with COVID, but other respiratory pathogens,” said Dr. Paul Griffin. There are some promising intranasal vaccination clinical trials.
I also think skin-patched vaccines will be crucial to our plan. They have many fascinating features, including long-term stability at room temperature.”
Platform Tech
Dr. Babaji Yadav said: “Platform technologies can certainly be used to leverage some of the clinical safety and efficacy data established with your prototype vaccines to support or accelerate the clinical development of your modified vaccines or subsequent vaccines. Platform technology delivers antigens and boosts immunogenicity.
At Novotech, where I work with several clients, regulatory agencies around the world have accepted this approach for mRNA, DNA, and even personalized cancer vaccines that used plasmid DNA or lipid nanoparticles as platform technologies.
In a guidance on public health emergency use of vaccines, USFDA states that if you are developing a modified vaccine against a new viral strain and keep the manufacturing process the same, you can submit an abbreviated data package with just the pharmacology and immunogenicity studies without having to repeat toxicology studies, which can take time and cost money.
Accelerating Trials: Patient Recruitment
Jenny Arellano said: The Asia Pacific region has over 4 billion people, 60% of the world’s population. Many participants are treatment-naive. Vaccine studies benefit from this and a varied population. Vaccine research requires a big study population and rich trial experience in these countries.
The region has good recruitment success, retention of clinical trial participants, and protocol-required visit compliance. The region is also appealing for vaccination studies due to legislative reform, increased investment in trial infrastructure, and cheaper clinical trial costs.
