Without medication pricing discussion, the gene-therapy revolution could stall 2023

“We wish to suggest a structure for the salt of deoxyribose nucleic acid (D.N.A.),” wrote James Watson and Francis Crick in 1953 (Nature 171, 737–738; 1953). “This structure has novel biologically interesting features.”
Researchers have spent 70 years unraveling those traits and using them for therapy. The outcome is a growing understanding of disease genetics and several treatments.
2023 may be a landmark in 70 years. CRISPR–Cas9 gene editing in somatic cells may be approved this year. Scientists and soon clinicians can alter selected genome areas to “correct” disease-causing genes. In the coming months, US, EU, and UK regulators will decide on a sickle-cell disease treatment that uses this strategy.

Even as gene editing develops, researchers worry about its future use in disease treatment. Gene therapies are too expensive for many who need them. Pricey gene-therapy research may deter government funding. Research institutions would find it harder to recruit outstanding people. Health economists must rapidly cooperate with industry and governments to establish a cheaper funding paradigm.
Million-dollar procedures
Years of steady improvements in virus-based gene therapy paved the way for CRISPR–Cas9. CAR-T-cell gene therapies, which create immune cells to treat cancer, have been approved by regulators in the previous decade. Clinical trials involve hundreds.
These therapies cost around US$1 million each treatment, plus hospital stays and cell manipulation procedures. Last year, the FDA approved the first gene treatment for haemophilia B, a genetic blood clotting disorder. Hemgenix, the most expensive medicine, costs $3.5 million each treatment.
Gene therapies cost more to develop and produce than small-molecule medication therapies. Gene treatments may offer a cure, freeing patients from pricey medications and hospitalizations. If a therapy saves millions in downstream treatments, the high cost may be justified.
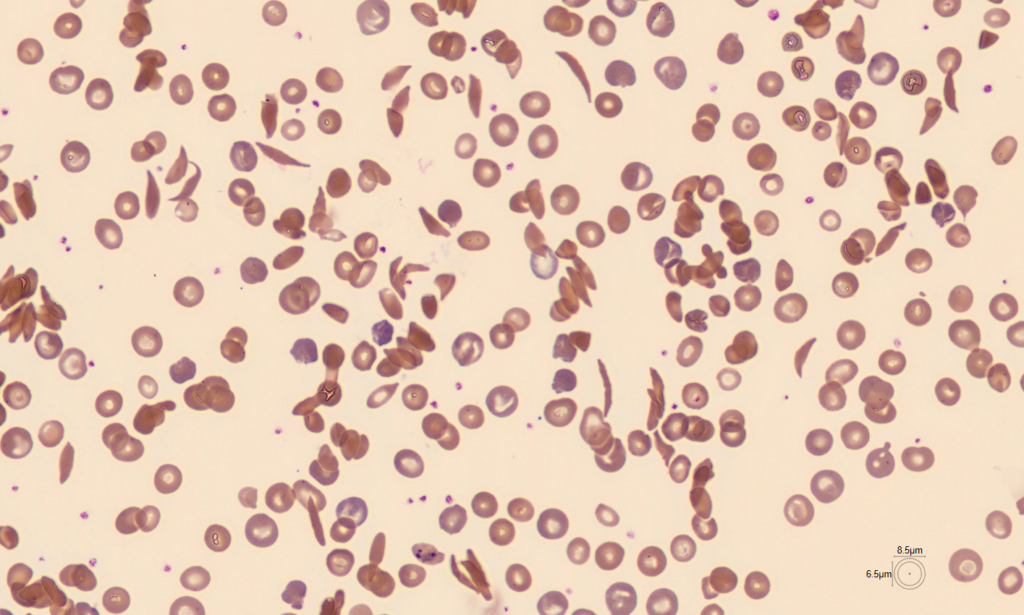
After all, conventional treatments pile up: one study indicated that treating a sickle-cell anemia patient in the US until age 64 costs $1.7 million (K. M. Johnson et al. Blood Adv. 7, 365–374; 2023).
Researchers applaud $3.5-million haemophilia gene therapy—but doubts remain.
Gene therapies are expensive, even in wealthy nations. After failing to negotiate a price with European regulators, Somerville, Massachusetts-based Bluebird Bio canceled plans to offer a gene therapy for β-thalassaemia in Europe in 2021. It planned to sell in the US, where drug prices are less regulated.
Even in America, costs matter. Steven Pearson, president of Boston’s Institute for Clinical and Economic Review, a health-economics think tank, says employers often subsidize US health insurance, and some are already saying they will likely restrict gene therapy coverage next year.
Low- and middle-income countries are completely abandoned. Given that β-thalassaemia and sickle-cell disease are more common in poorer countries, this is especially painful. Sickle-cell disease affects 2% of children in sub-Saharan Africa. Given the limited screening, this is likely an underestimate.
Improving access
Vertex Pharmaceuticals in Boston and CRISPR Therapeutics in Cambridge, Massachusetts, have not disclosed the cost of the CRISPR–Cas9 sickle-cell disease medication. Researchers expect the price tag.
At the March Third International Summit on Human Genome Editing in London, gene-editing therapies for low- and middle-income countries were discussed. Technology was used to speed treatment manufacture and testing. For sickle-cell treatment, clinicians isolate and modify blood-forming stem cells, destroy the rest, then reinfuse the edited cells. This may be cheaper and more accessible if it were a genome-editing operation done in the body rather than in isolated cells.
Develop safe and effective gene-therapy platforms. Gene-therapy developers may then swap in a gene that targets the disease without the safety and efficacy evaluations needed when beginning from scratch.
These technological solutions are limited. Pearson thinks US medicine pricing is unrelated to production costs since corporations can charge whatever the market would tolerate. Intellectual property rights and the difficulty of creating generic versions of biological treatments like gene therapy may limit price drops in other nations.
It is unknown how far academic centers can develop and implement gene treatments without pharmaceutical corporations’ financial resources and regulatory competence.
Gene-therapy debates include cost, regulation, and intellectual property. These will influence how far researchers may capitalize on Watson and Crick’s findings. Scientists must participate in these debates and advance them quickly.

